
This List can be Used to Learn the Names of all Elements in the Periodic Table. An atom or a nucleus of iron (chemical symbol Fe), for example, may be written 26 Fe. In this notation, the atomic number is not included. A list of 118 Elements and Their Symbols and Atomic Numbers is Provided in this Article. In the symbol representing a particular nuclear or atomic species, the atomic number may be indicated as a left subscript. Symbol-mass format for the above atom would be written as Cr-52. For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen.
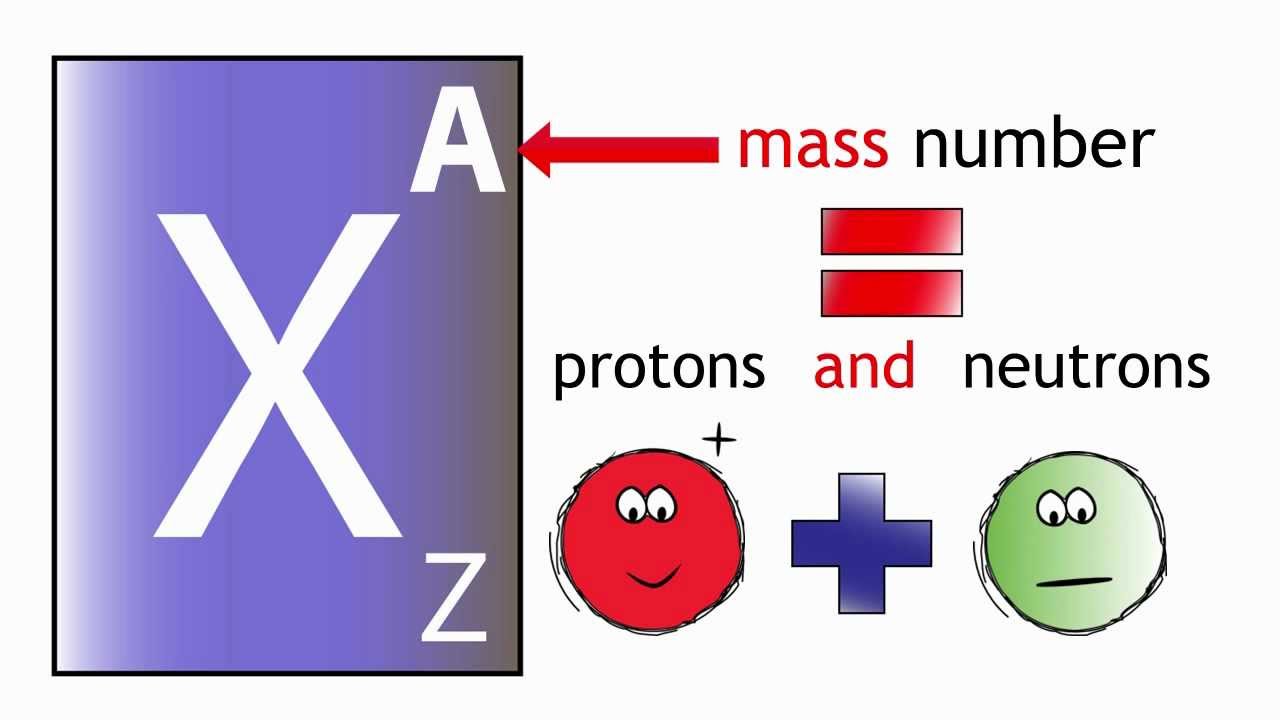
The number of protons in the nucleus is represented by Z, the atomic. The "A" value is written as a superscript while the "Z" value is written as a subscript. The illustration below shows the chemical symbol for the hypothetical element X. Both the atomic number and mass are written to the left of the chemical symbol. The symbol for an atom indicates the element via its usual two-letter symbol, the mass number as a left superscript, the atomic number as a left subscript (. The composition of any atom can be illustrated with a shorthand notation called A/Z format.


 0 kommentar(er)
0 kommentar(er)
